

 Now Location:Home--News Center--Company News
Now Location:Home--News Center--Company News
Multiple national standard products such as HLA and HPA, including Weihe, have participated in calibration
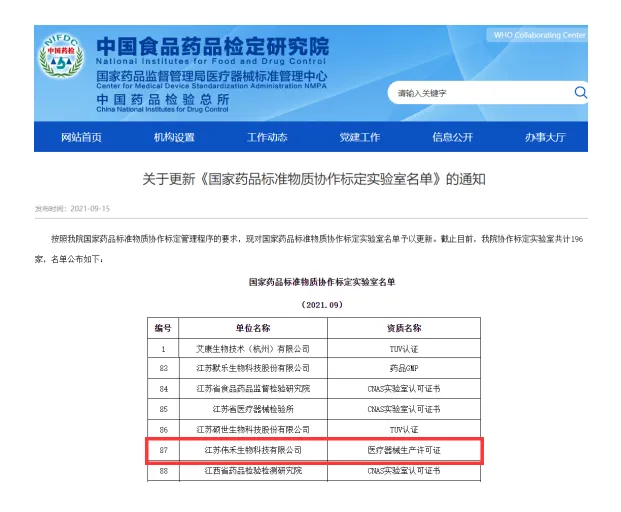
As a representative of the supply of molecular diagnostic products, Weihe Biotechnology has assisted the China Food and Drug Control Research Institute in formulating multiple standards, including the national reference for human leukocyte antigen HLA-B27 nucleic acid testing and the national reference for HLA-B * 5801/5701/1502 nucleic acid testing. This is also the first time that the China Inspection Institute has released standard products for the above-mentioned products. In addition to the completed standard materials, Weihe Biotechnology is currently actively cooperating with the China Food and Drug Control Research Institute to implement the development project of human platelet specific antigen standard materials, contributing its own corporate strength to precise blood matching.
Despite being monopolized by imported products for a long time in the fields of precision blood transfusion, transplantation and matching, Weihe Biotechnology has been deeply involved in this professional field for many years. As one of the few production enterprises, it can also stand out when competing with high-precision and cutting-edge import leading enterprises. The inclusion of Weihe Biotechnology in the list of national standard product agreement laboratories this time is a great encouragement for domestic enterprises to conduct independent research, a full affirmation of Weihe Biotechnology's product innovation ability, a hardcore test of the accuracy of results, and a recognition of the company as an industry newcomer in molecular fields such as precision blood transfusion and transplantation matching.
Weihe Biotechnology will forge ahead and make unremitting efforts to completely replace import monopolies in the future, achieve localization of molecular products such as precision blood transfusion and transplantation, and provide more diverse and cost-effective product choices for colleagues.
