

 Now Location:Home--News Center--Industry News
Now Location:Home--News Center--Industry News
Based on the breakthrough progress made in clinical trials of hematomas, we believe that CAR-T therapy as the fifth largest treatment for cancer is a high probability event. It is expected that the potential market space for hematomas in the United States will reach 48 billion US dollars per year (calculated at 300000 US dollars per person), and the potential market size for hematomas in China will reach 25 billion RMB (calculated at 100000 yuan per person).
CAR-T is a very personalized therapy, and the supply method of the product is fundamentally different from traditional drugs. Therefore, achieving standardization of CAR-T products is the primary task, which means controllable efficacy and risk. The future market will definitely belong to enterprises that can differentiate CAR-T cells, refine and standardize treatment processes. Hospitals or enterprises that are currently undergoing extensive development will inevitably be eliminated in the future.
CAR-T therapy has extremely strong technical attributes and strong replicability, which is significantly different from traditional new drug development. For China, where new drug development is just starting, there is a possibility of overtaking in the curve. At present, the number of CAR-T clinical trials conducted in China has reached 19, second only to the United States. This is also the first time that China has taken the international lead in the field of new drug research and development.
We believe that the common immune cells (NK, DC, CIK, etc.) currently used for cancer treatment will gradually withdraw from clinical treatment in the future. However, due to the fact that these types of immune cells can objectively enhance the human immune system and are all autologous cell expansions, the risk of reinfusion is relatively low, and they are expected to gain a large market in the fields of health care and adjuvant therapy.
Risk warning: For CAR-T therapy research and development enterprises, the risk comes from adverse clinical progress; For ordinary immune cell companies, the risk comes from strict regulation and medical insurance fee control.
The core of tumor immunotherapy is to overcome immune evasion
1.1Immune editing theory
Tumors are products of malignant transformation of normal cells in the body, characterized by continuous proliferation and metastasis within the body. Therefore, the prominent feature of tumor cells in immunology is the appearance of new antigen markers that cannot be seen in normal cells of the same type. The tumor antigens that have been gradually discovered include tumor specific antigens and tumor associated antigens. The former is unique to tumor cells; The latter mostly refers to embryonic antigens, which are shared between embryonic tissue and tumor tissue. Due to the presence of tumor antigens, they are inevitably recognized by the body's immune system and trigger specific immune responses, including cellular immunity and humoral immunity. The humoral immunity of tumors mainly involves the destructive effect of anti-tumor antibodies on tumor cells; Cellular immunity mainly refers to the killing effect of immune cells such as T lymphocytes, CIK cells, NK cells, and macrophages on tumor cells.
Tumor cells exhibit gene mutations and overexpression of oncogenes, and the proteins involved in these mutations or abnormal gene expressions are the basis for immune cells to recognize cancer cells. In theory, immune cells can eliminate abnormal cells at any time, thereby eliminating tumors in their embryonic state, also known as "immune surveillance". However, immune surveillance cannot completely prevent the occurrence of malignant tumors, and once a tumor develops, its malignancy gradually increases with the progression of the disease, ultimately leading to widespread metastasis.
In 2002, American oncobiologist R D. Schreiber proposed the theory of "tumor immune editing". According to this theory, the immune system not only has the ability to eliminate tumor cells, but also has a promoting effect on tumor growth. The occurrence and development of cancer cells in the body is a series of dynamic and complex interactions between the immune system and cancer cells. In this process, while the immune system clears some tumor cells, it also reshapes the biological characteristics of other tumor cells (such as tumor antigenicity), known as "immune editing". Tumor cells that have been immune edited are becoming increasingly malignant, and their resistance to immune attacks is becoming stronger, ultimately destroying the body's immune system, causing malignant growth and spread of tumor cells.

Tumor immune editing theory
1.2Traditional adoptive immune cells
Cancer Immunotherapy is a new treatment method that has emerged after surgery, chemotherapy, radiotherapy, and targeted therapy, and is known as the "fifth largest therapy" for treating cancer. It utilizes the power of the patient's own immune system to resist cancer. Immunotherapy has undergone years of continuous development and has achieved more and more encouraging research results in recent years. In 2013, cancer immunotherapy was ranked as the top ten scientific breakthroughs of the year by the top international academic journal Science.
Tumor immunotherapy can be broadly divided into two categories: non-specific and tumor antigen-specific. Non specific methods include immune checkpoint blockade and non-specific activation of immune cells; The main methods for tumor antigen specificity are various tumor vaccines and adoptive immune cell therapies.
Tumor adoptive immunotherapy is the induction, activation, and amplification of precursor cells of self or allogeneic anti-tumor effector cells in vitro using activators such as IL-2, anti-CD3 monoclonal antibodies, and specific peptides, and then infusing them back to tumor patients to enhance their anti-tumor immunity, in order to achieve the goal of treatment and prevention of recurrence. There are several common ones as follows. LAK cells activate peripheral blood mononuclear cells from patients or normal donors with high concentrations of IL-2. TIL cells are isolated from excised tumor tissue or cancerous pleural and ascitic fluid, and activated and expanded in vitro through IL-2 induction. DC-CIK cells are co cultured with CIK cells, which can regulate each other to increase cytokine release and enhance cytotoxicity, thereby significantly improving the proliferation and killing activity of CIK cells. The clinical treatment effect is superior to other effector cells such as CIK. CTL cells were induced to clone in vitro using specific peptide antigens.
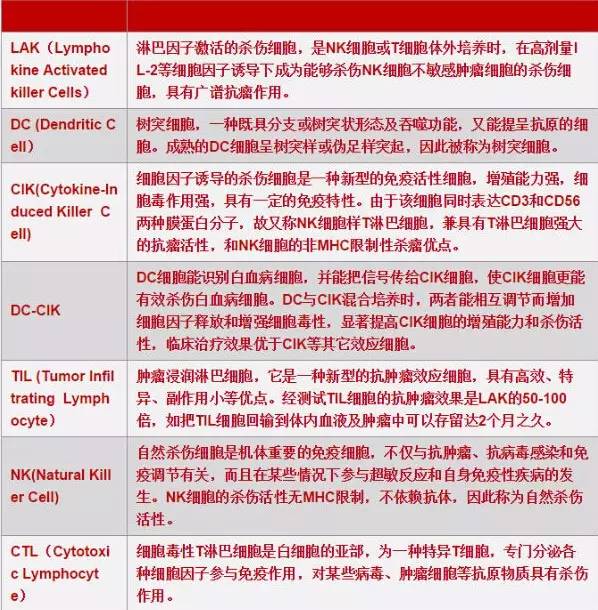
Traditional adoptive immune cells
At present, nearly 200 companies in China are using traditional adoptive immune technology for clinical cancer treatment. The general business model is to provide immune cell therapy technology services to medical institutions and charge technical service fees. The company dispatches professional technical personnel to the designated cell laboratory of the hospital, and according to the immune cell treatment plan formulated by the hospital for tumor patients, uses its self-developed patented immune cell treatment technology to separate and culture the patient's blood samples collected by the hospital, expand them in vitro, and return them to the hospital; The hospital will administer the cell preparation back into the patient's body. The hospital pays the company's technical service fees, but the company does not participate in the diagnosis and treatment plan determination of patients, nor does it directly charge patients. It only serves as a technology supplier for the in vitro cell culture process.

Immune cell therapy process
Although various traditional adoptive immune cells have certain effects on cancer, their targeting ability is poor and it is often difficult to show significant therapeutic effects. They are mostly used for adjuvant therapy or postoperative immune enhancement, gradually being eliminated by mainstream technologies in Europe and America. However, due to the fact that these types of immune cells can objectively enhance the human immune system and are all expanded from autologous cells, the risk of reinfusion is relatively low, and they are expected to gain a large market in the fields of health care and adjuvant therapy in the future.
Currently, countries around the world are actively developing CAR-T treatment technology and conducting clinical trials, including the United States, China, the United Kingdom, Sweden, Japan, and others. International pharmaceutical giant Novartis is the first to collaborate with the University of Pennsylvania to develop research on CAR-T cell immunotherapy. Subsequently, pharmaceutical giants such as Lilly, GlaxoSmithKline, and Pfizer, as well as small biopharmaceutical companies such as Juno and Kite, also competed to join the research and development of CAR-T cell immune technology. This will undoubtedly promote the early entry of this highly promising tumor immunotherapy technology into the clinical application stage, thus bringing hope to patients with advanced refractory malignant tumors.
1.3 CAR-T:Taking over the banner of cancer immunotherapy
T lymphocytes are the natural enemies of tumor cells and play a major role in the tumor immune response, with extremely strong killing effects on tumor cells. However, when using endogenous T cells for tumor immunotherapy, the target antigen needs to be processed before it can interact with the main histocompatibility complex (MHC) on the surface of the target cells, which is known as MHC restriction. However, the process of tumor immune editing can lead to a decrease in MHC expression on the surface of tumor cells, disrupt antigen processing, and reduce peptide immunogenicity. This long-term immune escape mechanism can enable tumor cells to successfully evade T cell attacks and rapidly proliferate. In addition, the number of tumor specific T cells in the human body is relatively small, and due to the continuous expression of autoantigens by most tumor cells, T cells targeting these antigens are neutralized or removed through immune tolerance mechanisms, further reducing the number. Therefore, although T cell adoptive immunotherapy, including cytokine induced killer cells, has achieved certain results in the treatment of some tumors, its efficacy is still unsatisfactory in most tumors.
CAR-T, also known as ChimericAntigen Receptor T-Cell Immunotherapy, stands for Chimeric Antigen Receptor T-Cell Immunotherapy. By identifying tumor associated antigen (TAA), which refers to some glycoproteins or glycolipid components on the surface of tumor cells that are expressed in trace amounts on normal cells but significantly increased in tumor cells, scFv and the intracellular signal domain "immune receptor tyrosine based activation motifs (ITAM), usually CD3," ζ Or Fc ε RI γ)” Gene recombination is performed in vitro to generate recombinant plasmids, which are then transfected into the patient's T cells using transfection technology in vitro to express tumor antigen receptors. After purification and large-scale expansion after transfection, T cells, also known as CAR-T cells, can specifically recognize tumor related antigens, significantly improve the targeting, killing activity, and persistence of effector T cells compared to conventional immune cells, and overcome the local immune suppression microenvironment of tumors, thereby breaking the host's immune tolerance state and killing tumor cells.
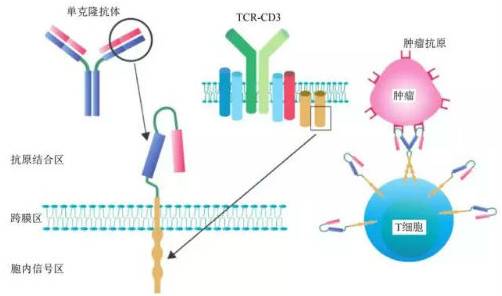
The complete CAR-T structure consists of three parts: antigen binding region, transmembrane linking region, and intracellular signaling region.
1.3.1Fourth generation CAR-T technology evolution
Research has shown that the complete activation of T cells depends on the dual signaling and cytokine effects. The first signal is a specific signal, initiated by TCR recognition of antigen peptide MHC complexes on the surface of antigen-presenting cells; The second signal is a co stimulatory signal, which promotes IL-2 synthesis through important co stimulatory molecules such as CD28/B7, and fully activates T cells to prevent apoptosis. For the initial type of T cells (T cells that do not come into contact with antigens), it is impossible for T cells to function normally under the condition of signal 1 and no signal 2; Even if T cells come into contact with antigens, they cannot function normally without co stimulatory signals. Correspondingly, only containing CD3 ζ The chimeric antigen receptor of the sequence, without co stimulatory signal 2, cannot activate CAR-T cells. Therefore, according to the dual signal theory of T cell activation, the second and third generation CARs add co stimulatory molecules (CM) such as CD28, CD134 (OX40), and CD137 (4-1BB) to chimeric receptors, with the aim of increasing co stimulatory factors to enhance T cell cytotoxicity and proliferation activity, maintain T cell response, and prolong T cell survival time, thereby enhancing the effectiveness of CAR-T.
Changes in intracellular signaling regions of third-generation CAR-T cells
The fourth generation CAR-T technology currently under development includes integrating expression of immune factors, integrating co stimulatory factor ligands, and so on. It can be imagined that there will be more co stimulatory factors and other precise regulatory methods added in the future, which also highlights the technical attributes of CAR-T.
1.3.2 CAR-TTreatment process
A typical CAR-T treatment process is mainly divided into the following five steps.
①Separation: Separate immune T cells from cancer patients.
②Modification: Using genetic engineering technology to add a chimeric antibody that can recognize tumor cells and activate T cells simultaneously to prepare CAR-T cells.
③Amplification: In vitro culture, extensively amplifying CAR-T cells. Generally, a patient requires billions, or even tens of billions, of CAR-T cells (the larger the body size, the more cells needed).
④Infusion: Infuse the amplified CAR-T cells back into the patient's body.
⑤Monitoring: closely monitor the patient, especially controlling the intense physical reactions in the past few days.
The entire treatment course lasts for about 3 weeks, and the process of cell extraction, modification, and expansion takes about 2 weeks, which takes a longer time.
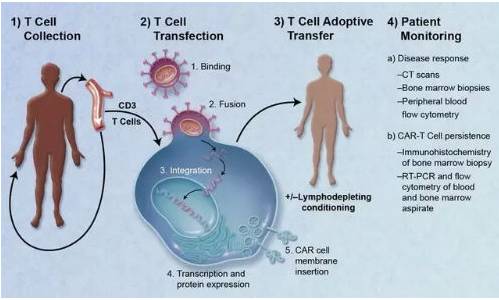
CAR-TTreatment process
1.3.3 CAR-TClinical progress
CAR-T therapy was pioneered by Professor Carl June, a tenured professor at the University of Pennsylvania and a member of the American Academy of Sciences, along with his team. Later, more and more scientists, hospitals, and pharmaceutical companies participated in the development of CAR-T technology. With the continuous breakthrough progress, more and more investment institutions have also seen the prospects of this therapy, and the continuous influx of funds has accelerated the advancement of technology.
When it comes to the clinical progress of CAR-T, the first thing to mention is the story of Emily Whitehead. At the age of 5, Emily was diagnosed with acute lymphocytic leukemia and was infected during the first round of chemotherapy, almost losing both legs. Later, her condition recurred, and she received treatment and was scheduled to undergo bone marrow transplantation surgery. During the waiting period, the condition recurred again, and the doctors were at a loss. Subsequently, receiving CART19 cell reinfusion from Carl June, Emily experienced a strong immune response in her body and developed a high fever for several consecutive days, requiring hospitalization for treatment. Under the high fever, she had an illusion and asked her father, "How come there is a pond in my room?" After experiencing a nightmare like continuous high fever, Emily finally broke free from the constraints of death and woke up again. Emily is now 9 years old and still growing up healthy. Surviving CAR-T cells can still be detected in her body.

CAR-T therapy inventor Carl June and cured Emily Whitehead
The article "The Dizzying Journal to a New Cancer Army" published in Science provides a detailed record of Carl June and his team's research and development process on CAR-T clinical trials. The excerpt about Emily is as follows Emily Whitehead, a 6-year old with end stage Leukemia who parents turned to June's cell therapy as a last ditch hope The experiential treatment sent her body into a dead urgent drive She spent 2 weeks on average in the CHOP intensive care unit while doctors tried everything they wanted to save her "We thought it was over," June said He drafted an email message to Penn's proposal: "It is with register that I inform you that our first pediatric patient on the CART19 trial will like die," he wrote "There is nothing to do at this point other than hope for amide." (Science. 2013.) 340. 1514-1518)
Since last year, new developments in CAR-T clinical practice and the development of new technologies have been heard almost every month, even every week. A small part of it is now extracted as follows.
On December 10, 2014, at the 56th Annual Meeting of the American Society of Hematology (ASH), Novartis released its latest clinical data on CAR-T immunotherapy CTL019. In these studies, CTL019 has shown great therapeutic potential in certain types of lymphocytic leukemia. In a long-term pediatric study, 39 pediatric patients with relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL) received treatment with CTL019. Data showed that 36 patients experienced complete remission (CR), accounting for 92% (n=36/39).
On May 21, 2015, at the 10th World Congress of Stem Cells and Regenerative Medicine held in London, UK, Cao Wei, CEO of Cellular Biomedicine Group, released phase I clinical data for its CAR-TCD30 Hodgkin lymphoma immune tumor development project. 5 out of 7 patients responded to CAR-CD30 T cell therapy. The experiment was designed and executed by the General Hospital of the People's Liberation Army of China, with Professor Han Weidong, the director of the cancer immunotherapy department at the hospital, as the main project leader.
On June 2, 2015, at the annual meeting of the American Society of Clinical Oncology (ASCO), the Memorial Sloan Kettering Cancer Center (MSKCC) announced the results of its Phase I clinical trial of CAR-T cell therapy for recurrent and refractory B-cell non Hodgkin's lymphoma. Eight patients received treatment after high-dose chemotherapy and autologous hematopoietic stem cell transplantation, of which five patients achieved complete remission (62.5%). Some patients experienced cytokine storms during this period and were effectively controlled through the use of tocilizumab combined with corticosteroid therapy.
Professor Carl June stated in an interview this year that the CAR-T technology began its first clinical trial in 2010 and is estimated to have been approved by the US FDA in 2017, taking only 7 years. This is very fast.
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
