

 Now Location:Home--News Center--Company News
Now Location:Home--News Center--Company News
The clock is set to the last day of 2019 in a blink of an eye. While lamenting the passage of time and the speed of time, at this important time, we are accustomed to looking back at the path we have walked in the past.
This year, Weihe has laid a solid foundation in the HPA market and is steadily advancing. Following the HPA demonstration experiments in previous provinces, Weihe firmly grasped the tail of 2019 and completed a comparative demonstration tour of HPA in three blood centers in just the last month, namely Tianjin Blood Center, Nanjing Red Cross Center, and Shaanxi Blood Center.
Our company's comparative demonstration experiments are all blind screens for comparative verification experiments. Among them, the Tianjin Blood Center completed a blind screening comparison experiment for 30 samples of HPA on the Roche Z480 fluorescence quantitative PCR instrument platform. The Nanjing Red Cross Center, under the guidance of teachers from the Blood Transfusion Research Room, completed the combined reagent kit sample testing of HPA and HLA-A/B. The Shaanxi Provincial Blood Center conducted experimental comparison testing on our HPA reagents on the Roche LC480 instrument platform. Through comparative demonstration experiments, the sample record results of the three blind screening methods mentioned above are exactly the same as those reported by Weihe qPCR methodology. The high efficiency, convenient operation, and accuracy of Weihe platelet gene bank reagents have been unanimously recognized and praised by teachers from various units.


The picture shows a demonstration experiment of HPA and HLA combined reagents conducted at the Nanjing Red Cross Center

The picture shows the operational site of HPA testing at the Shaanxi Provincial Blood Center
The demonstrations at the three blood centers in Tianjin, Nanjing, and Shaanxi marks a small conclusion for Weihe in the busy December of 2019. Next, Weihe will first complete comparative experiments with some intended customers, including Shanghai Blood Center, Fujian Blood Center, and Shenzhen Blood Center. In 2020, they will embark on a new journey, expand their market, cover a wider range of regions, and truly promote the platelet gene bank project to clinical practice, achieving full coverage of matching for patients with adverse transfusion reactions, and achieving a benign mechanism where there is a database to follow and a database to use.
Our company has focused on promoting platelet gene typing products this year. Due to their close proximity to the blood station system and clinical needs, the testing efficiency has been greatly improved, and they are convenient to use with significant advantages. We have received more than 20 potential customers for cooperation, successfully demonstrated 15 provincial-level blood centers, and successfully completed transactions with 9 customers. So far, our company has successfully analyzed tens of thousands of HPA samples, compared thousands of samples, and is compatible with most fluorescence quantitative PCR instruments on the market. We have received high praise from customers who have completed transactions and demonstrations. Regarding the more than ten samples collected during the sample analysis process that had doubts about the comparison of customer results, our company conducted sequencing review and testing. After comparison, the sequencing results of the questionable samples were completely consistent with the testing results of our company's products, which also reflects the high accuracy and compatibility of the Weihe reagent kit, achieving the goal of zero errors in platelet gene bank construction reagents.
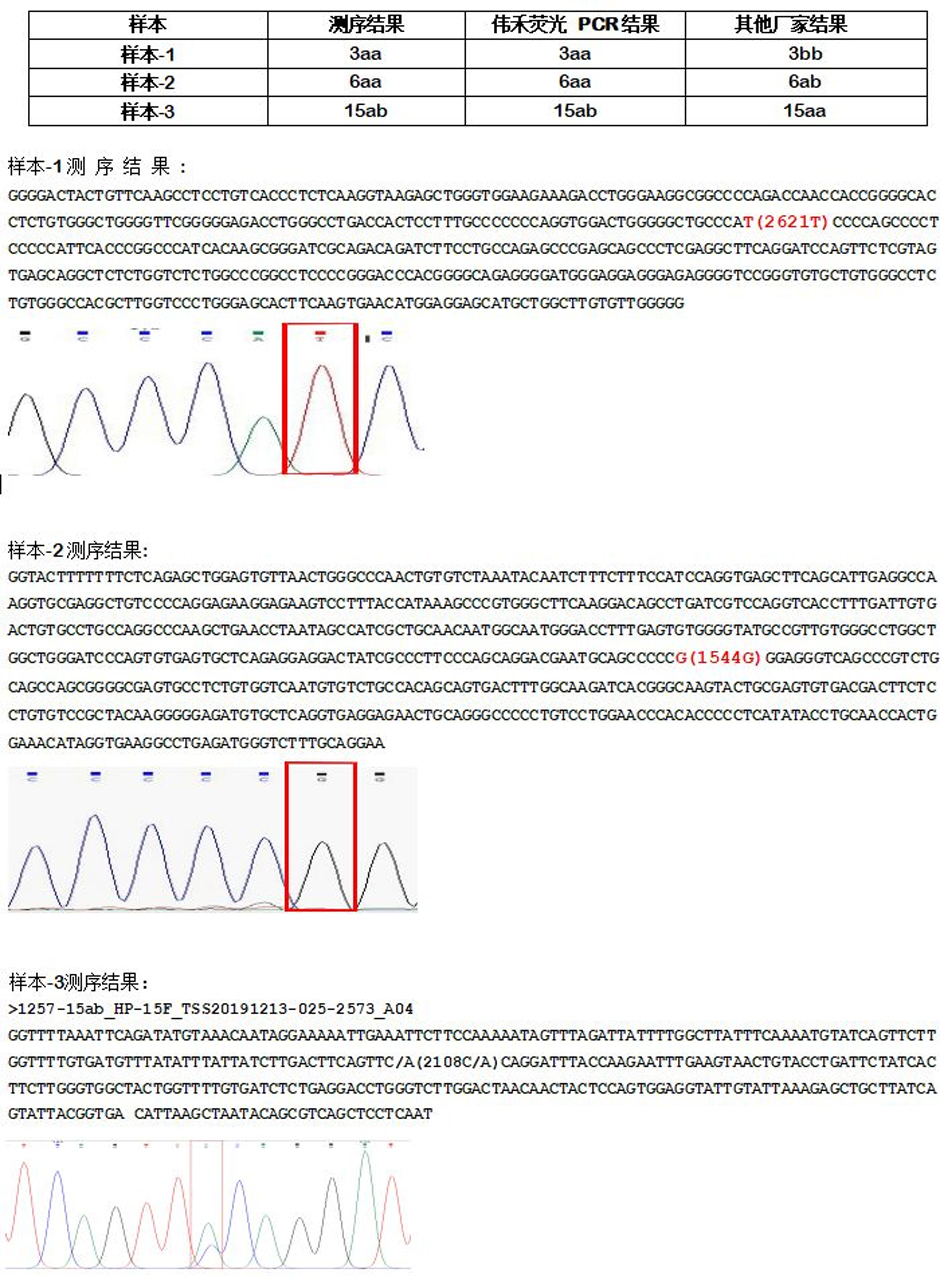
The sequencing analysis results of some questionable samples in the figure are consistent with the results of Weihe multiplex fluorescence
The philosophy of Weihe has never been to conquer the vast sea of stars, defeat competitors, or occupy large markets, which is not our original intention. Our hope is that when you encounter any problems or needs, we can provide assistance behind the scenes and silently shine for scientific research experiments. Next, in order to cooperate with the efficient promotion of the platelet matching infusion project of the China Blood Transfusion Association, our company will continue to strive for professionalism in product services, differentiation of customer needs, and strategic project cooperation, and steadily move forward. In 2020, we will be even better!
[Contact Information]
Address: 225300, East Side, 2nd Floor, Building G75, Phase 4, Yaocheng, Medical High tech Zone, Taizhou City, Jiangsu Province
Phone: 0523-86201335-8010 (product consultation)
Website: www.wehelpinc.com
Email: sales@wehelpinc.com
